Once Again What Is the Function of the Fully Developed Endometrium
| Endometrium | |
|---|---|
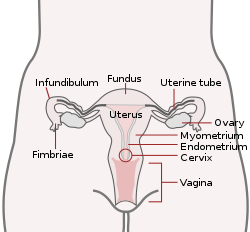 Uterus and uterine tubes. (Endometrium labeled at center right.) | |
 Endometrium in the proliferative phase | |
| Details | |
| Role of | Uterus |
| Identifiers | |
| Latin | tunica mucosa uteri |
| MeSH | D004717 |
| TA98 | A09.1.03.027 |
| TA2 | 3521 |
| FMA | 17742 |
| Anatomical terminology [edit on Wikidata] | |
The endometrium is the inner epithelial layer, along with its mucous membrane, of the mammalian uterus. Information technology has a basal layer and a functional layer; the functional layer thickens and and so is shed during menstruum in humans and some other mammals, including apes, Onetime World monkeys, some species of bat, the elephant shrew[1] and the Cairo spiny mouse.[2] In nigh other mammals, the endometrium is reabsorbed in the estrous bike. During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse and get interconnected, forming the placenta, which supplies oxygen and nutrition to the embryo and fetus.[three] [4] The speculated presence of an endometrial microbiota[5] has been argued confronting.[vi] [7]
Structure [edit]
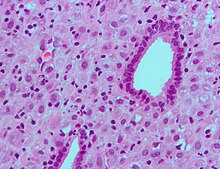
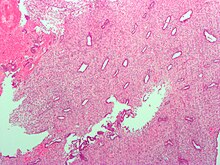
Low magnification micrograph of decidualized endometrium. H&East stain
The endometrium consists of a single layer of columnar epithelium plus the stroma on which it rests. The stroma is a layer of connective tissue that varies in thickness according to hormonal influences. In the uterus, simple tubular glands reach from the endometrial surface through to the base of operations of the stroma, which as well carries a rich claret supply provided past the spiral arteries. In a woman of reproductive historic period, ii layers of endometrium can be distinguished. These 2 layers occur only in the endometrium lining the cavity of the uterus, and non in the lining of the Fallopian tubes.[3] [four]
- The functional layer is adjacent to the uterine crenel. This layer is congenital up after the cease of flow during the offset part of the previous menstrual bicycle. Proliferation is induced past estrogen (follicular phase of menstrual bike), and later changes in this layer are engendered past progesterone from the corpus luteum (luteal phase). It is adapted to provide an optimum environs for the implantation and growth of the embryo. This layer is completely shed during catamenia.
- The basal layer, adjacent to the myometrium and below the functional layer, is not shed at whatever time during the menstrual cycle. The functional layer develops on summit of it.
In the absence of progesterone, the arteries supplying blood to the functional layer tuck, so that cells in that layer become ischaemic and die, leading to menstruation.
It is possible to identify the phase of the menstrual cycle past reference to either the ovarian cycle or the uterine bicycle past observing microscopic differences at each phase—for example in the ovarian bike:
| Stage | Days | Thickness | Epithelium |
|---|---|---|---|
| Menstrual phase | one–5 | Thin | Absent-minded |
| Follicular phase | 5–14 | Intermediate | Columnar |
| Luteal phase | 15–27 | Thick | Columnar. Also visible are arcuate vessels of uterus |
| Ischemic stage | 27–28 | Columnar. Likewise visible are arcuate vessels of uterus |
Gene and protein expression [edit]
Nigh 20,000 poly peptide coding genes are expressed in human cells and some 70% of these genes are expressed in the normal endometrium.[8] [ix] Just over 100 of these genes are more than specifically expressed in the endometrium with only a scattering genes being highly endometrium specific. The corresponding specific proteins are expressed in the glandular and stromal cells of the endometrial mucosa. The expression of many of these proteins vary depending on the menstrual wheel, for case the progesterone receptor and thyrotropin-releasing hormone both expressed in the proliferative stage, and PAEP expressed in the secretory phase. Other proteins such as the HOX11 protein that is required for female fertility, is expressed in endometrial stroma cells throughout the menstrual bike. Certain specific proteins such as the estrogen receptor are also expressed in other types of female tissue types, such as the cervix, fallopian tubes, ovaries and breast.[10]
Microbiome speculation [edit]
The uterus and endometrium was for a long time thought to be sterile. The cervical plug of mucosa was seen to foreclose the entry of any microorganisms ascending from the vagina. In the 1980s this view was challenged when it was shown that uterine infections could ascend from weaknesses in the bulwark of the cervical plug. Organisms from the vaginal microbiota could enter the uterus during uterine contractions in the menstrual cycle. Further studies sought to identify microbiota specific to the uterus which would be of help in identifying cases of unsuccessful IVF and miscarriages. Their findings were seen to exist unreliable due to the possibility of cross-contamination in the sampling procedures used. The well-documented presence of Lactobacillus species, for case, was hands explained past an increase in the vaginal population beingness able to seep into the cervical mucous.[half dozen] Some other study highlighted the flaws of the earlier studies including cross-contamination. Information technology was too argued that the prove from studies using germ-free offspring of axenic animals (sanitary) conspicuously showed the sterility of the uterus. The authors ended that in lite of these findings there was no existence of a microbiome.[7]
The normal authorization of Lactobacilli in the vagina is seen as a marker for vaginal wellness. However, in the uterus this much lower population is seen every bit invasive in a closed surroundings that is highly regulated past female sex hormones, and that could have unwanted consequences. In studies of endometriosis Lactobacillus is not the dominant type and at that place are higher levels of Streptococcus and Staphylococcus species. Half of the cases of bacterial vaginitis showed a polymicrobial biofilm attached to the endometrium.[six]
Function [edit]
The endometrium is the innermost lining layer of the uterus, and functions to foreclose adhesions between the opposed walls of the myometrium, thereby maintaining the patency of the uterine cavity. During the menstrual cycle or estrous bike, the endometrium grows to a thick, blood vessel-rich, glandular tissue layer. This represents an optimal surroundings for the implantation of a blastocyst upon its arrival in the uterus. The endometrium is key, echogenic (detectable using ultrasound scanners), and has an average thickness of six.7 mm.
During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse and become interconnected, forming the placenta, which supplies oxygen and nutrition to the embryo and fetus.
Cycle [edit]
The endometrial lining undergoes cyclic regeneration. Humans, apes, and some other species display the menstrual cycle, whereas most other mammals are discipline to an estrous cycle.[1] In both cases, the endometrium initially proliferates under the influence of estrogen. Withal, in one case ovulation occurs, the ovary (specifically the corpus luteum) volition produce much larger amounts of progesterone. This changes the proliferative pattern of the endometrium to a secretory lining. Somewhen, the secretory lining provides a hospitable environment for one or more blastocysts.
Upon fertilization, the egg may implant into the uterine wall and provide feedback to the torso with man chorionic gonadotropin (HCG). HCG provides connected feedback throughout pregnancy past maintaining the corpus luteum, which will proceed its function of releasing progesterone and estrogen. The endometrial lining is either reabsorbed (estrous wheel) or shed (menstrual wheel). In the latter case, the process of shedding involves the breaking downward of the lining, the tearing of small connective claret vessels, and the loss of the tissue and claret that had constituted information technology through the vagina. The entire process occurs over a flow of several days. Menstruation may be accompanied by a serial of uterine contractions; these help expel the menstrual endometrium.
In instance of implantation, however, the endometrial lining is neither absorbed nor shed. Instead, it remains as decidua. The decidua becomes part of the placenta; it provides back up and protection for the gestation.
If in that location is inadequate stimulation of the lining, due to lack of hormones, the endometrium remains thin and inactive. In humans, this will result in amenorrhea, or the absence of a menstrual period. Subsequently menopause, the lining is often described as being atrophic. In contrast, endometrium that is chronically exposed to estrogens, but not to progesterone, may become hyperplastic. Long-term utilise of oral contraceptives with highly potent progestins can as well induce endometrial cloudburst.[eleven] [12]
In humans, the cycle of building and shedding the endometrial lining lasts an average of 28 days. The endometrium develops at different rates in unlike mammals. Various factors including the seasons, climate, and stress tin affect its development. The endometrium itself produces certain hormones at dissimilar stages of the cycle and this affects other parts of the reproductive arrangement.
[edit]

Histopathologic and cytopathologic images.
(A) proliferative endometrium (Left: HE × 400) and proliferative endometrial cells (Right: HE × 100)
(B) secretory endometrium (Left: HE × 10) and secretory endometrial cells (Right: HE × 10)
(C) atrophic endometrium (Left: HE × 10) and atrophic endometrial cells (Correct: HE × 10)
(D) mixed endometrium (Left: HE × ten) and mixed endometrial cells (Right: HE × 10)
(E): endometrial atypical hyperplasia (Left: HE × 10) and endometrial atypical cells (Right: HE × 200)
(F) endometrial carcinoma (Left: HE × 400) and endometrial cancer cells (Right: HE × 400).
Chorionic tissue can outcome in marked endometrial changes, known every bit an Arias-Stella reaction, that have an appearance similar to cancer.[13] Historically, this alter was diagnosed as endometrial cancer and it is of import only in so far equally it should not be misdiagnosed as cancer.
- Adenomyosis is the growth of the endometrium into the muscle layer of the uterus (the myometrium).
- Endometriosis is the growth of endometrial tissue exterior the uterus.
- Endometrial hyperplasia
- Endometrial cancer is the most common cancer of the human female genital tract.
- Asherman'due south syndrome, besides known as intrauterine adhesions, occurs when the basal layer of the endometrium is damaged past instrumentation (e.m., D&C) or infection (due east.g., endometrial tuberculosis) resulting in endometrial sclerosis and adhesion formation partially or completely obliterating the uterine cavity.
Sparse endometrium may be defined as an endometrial thickness of less than viii mm. It usually occurs later menopause. Treatments that can improve endometrial thickness include Vitamin E, L-arginine and sildenafil citrate.[14]
Gene expression profiling using cDNA microarray can be used for the diagnosis of endometrial disorders.[15] The European Menopause and Andropause Society (EMAS) released Guidelines with detailed information to assess the endometrium. [16]
Embryo transfer [edit]
An endometrial thickness of less than 7 mm decreases the pregnancy charge per unit in in vitro fertilization by an odds ratio of approximately 0.4 compared to an EMT of over 7 mm. Nonetheless, such low thickness rarely occurs, and whatever routine use of this parameter is regarded every bit not justified.[17]

Triple-line endometrium measuring 7mm.
Observation of the endometrium past transvaginal ultrasonography is used when administering fertility medication, such as in in vitro fertilization. At the time of embryo transfer, information technology is favorable to have an endometrium of a thickness of betwixt 7 and 14 mm with a triple-line configuration,[18] which means that the endometrium contains a hyperechoic (usually displayed equally light) line in the middle surrounded by ii more hypoechoic (darker) lines. A triple-line endometrium reflects the separation of the stratum basalis and functionalis layers, and is too observed in the periovulatory period secondary to rising estradiol levels, and disappears later ovulation.[19]
Additional images [edit]
-

Endometrioid adenocarcinoma from biopsy. H&E stain.
-

Micrograph of decidualized endometrium due to exogenous progesterone. H&Eastward stain.
-

Micrograph showing endometrial stromal condensation, a finding seen in menses.
Run across likewise [edit]
- CYTL1, too known as cytokine-like like protein 1.
References [edit]
- ^ a b Emera, D; Romero, R; Wagner, Thousand (January 2012). "The evolution of menstruation: a new model for genetic absorption: explaining molecular origins of maternal responses to fetal invasiveness". BioEssays. 34 (i): 26–35. doi:10.1002/bies.201100099. PMC3528014. PMID 22057551.
- ^ Bellofiore, N.; Ellery, S.; Mamrot, J.; Walker, D.; Temple-Smith, P.; Dickinson, H. (2016-06-03). "Starting time prove of a menstruating rodent: the spiny mouse (Acomys cahirinus)". bioRxiv. 216 (1): 40.e1–40.e11. doi:10.1101/056895. PMID 27503621. S2CID 196624853.
- ^ a b Bluish Histology - Female Reproductive Organisation. School of Anatomy and Homo Biology — The University of Western Australia Accessed 20061228 20:35
- ^ a b Guyton Air conditioning, Hall JE, eds. (2006). "Chapter 81 Female Physiology Before Pregnancy and Female person Hormones". Textbook of Medical Physiology (11th ed.). Elsevier Saunders. pp. 1018ff. ISBN9780721602400.
- ^ Franasiak, Jason M.; Scott, Richard T. (2015). "Reproductive tract microbiome in assisted reproductive technologies". Fertility and Sterility. 104 (half-dozen): 1364–1371. doi:10.1016/j.fertnstert.2015.ten.012. ISSN 0015-0282. PMID 26597628.
- ^ a b c Baker, JM; Chase, DM; Herbst-Kralovetz, MM (2018). "Uterine Microbiota: Residents, Tourists, or Invaders?". Frontiers in Immunology. 9: 208. doi:10.3389/fimmu.2018.00208. PMC5840171. PMID 29552006.
- ^ a b Perez-Muñoz, ME; Arrieta, MC; Ramer-Tait, AE; Walter, J (28 April 2017). "A critical cess of the "sterile womb" and "in utero colonization" hypotheses: implications for research on the pioneer infant microbiome". Microbiome. 5 (1): 48. doi:10.1186/s40168-017-0268-4. PMC5410102. PMID 28454555.
- ^ "The human proteome in endometrium - The Human Poly peptide Atlas". world wide web.proteinatlas.org . Retrieved 2017-09-25 .
- ^ Uhlén, Mathias; Fagerberg, Linn; Hallström, Björn Thou.; Lindskog, Cecilia; Oksvold, Per; Mardinoglu, Adil; Sivertsson, Åsa; Kampf, Caroline; Sjöstedt, Evelina (2015-01-23). "Tissue-based map of the human proteome". Scientific discipline. 347 (6220): 1260419. doi:10.1126/science.1260419. ISSN 0036-8075. PMID 25613900. S2CID 802377.
- ^ Zieba, Agata; Sjöstedt, Evelina; Olovsson, Matts; Fagerberg, Linn; Hallström, Björn M.; Oskarsson, Linda; Edlund, Karolina; Tolf, Anna; Uhlen, Mathias (2015-10-21). "The Human being Endometrium-Specific Proteome Defined by Transcriptomics and Antibody-Based Profiling". OMICS: A Journal of Integrative Biological science. xix (11): 659–668. doi:10.1089/omi.2015.0115. PMID 26488136.
- ^ Deligdisch, Fifty. (1993). "Effects of hormone therapy on the endometrium". Modern Pathology. half-dozen (1): 94–106. PMID 8426860.
- ^ William's Gynecology, McGraw 2008, Chapter viii, Abnormal Uterine Haemorrhage
- ^ Arias-Stella, J. (Jan 2002). "The Arias-Stella reaction: facts and fancies four decades later". Adv Anat Pathol. 9 (1): 12–23. doi:10.1097/00125480-200201000-00003. PMID 11756756. S2CID 26249687.
- ^ Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura Chiliad, Sugino N (April 2010). "Endometrial growth and uterine blood flow: a pilot report for improving endometrial thickness in the patients with a sparse endometrium". Fertil. Steril. 93 (6): 1851–8. doi:10.1016/j.fertnstert.2008.12.062. PMID 19200982.
- ^ Tseng, L.; Chen, I.; Chen, M.; Yan, H.; Wang, C.; Lee, C. (2010). "Genome-based expression profiling as a single standardized microarray platform for the diagnosis of endometrial disorder: an array of 126-cistron model". Fertility and Sterility. 94 (i): 114–119. doi:10.1016/j.fertnstert.2009.01.130. PMID 19328470.
- ^ Dreisler E, Poulsen LG, Antonsen SL, Ceausu I, Depypere H, Erel CT, Lambrinoudaki I, Pérez-López FR, Simoncini T, Tremollieres F, Rees M, Ulrich LG (2013). "EMAS clinical guide: Assessment of the endometrium in peri and postmenopausal women". Maturita. 75 (2): 181–90. doi:x.1016/j.maturitas.2013.03.011. PMID 23619009.
- ^ Kasius, A.; Smit, J. One thousand.; Torrance, H. L.; Eijkemans, M. J. C.; Mol, B. W.; Opmeer, B. C.; Broekmans, F. J. M. (2014). "Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-assay". Human Reproduction Update. 20 (4): 530–541. doi:10.1093/humupd/dmu011. ISSN 1355-4786. PMID 24664156.
- ^ Zhao, Jing; Zhang, Qiong; Li, Yanping (2012). "The upshot of endometrial thickness and blueprint measured by ultrasonography on pregnancy outcomes during IVF-ET cycles". Reproductive Biological science and Endocrinology. 10 (1): 100. doi:10.1186/1477-7827-10-100. ISSN 1477-7827. PMC3551825. PMID 23190428.
- ^ Baerwald, A. R.; Pierson, R. A. (2004). "Endometrial development in association with ovarian follicular waves during the menstrual wheel". Ultrasound in Obstetrics and Gynecology. 24 (four): 453–460. doi:10.1002/uog.1123. ISSN 0960-7692. PMC2891966. PMID 15343603.
External links [edit]
- Beefcake figure: 43:05-15 at Homo Anatomy Online, SUNY Downstate Medical Center - "The uterus, uterine tubes and ovary with associated structures."
- Histology epitome: 18902loa – Histology Learning Arrangement at Boston University - "Female Reproductive System uterus, endometrium"
- Swiss embryology (from UL, UB, and UF) gnidation/role02
- Histology image: 20_01 at the University of Oklahoma Wellness Sciences Center
- Histology at utah.edu. Slide is proliferative phase - click forrard to run across secretory phase
Source: https://en.wikipedia.org/wiki/Endometrium
0 Response to "Once Again What Is the Function of the Fully Developed Endometrium"
Post a Comment